Hip Innovation Technology Product Testing
Cadaver Studies
A series of Cadaver Studies were performed with the Reverse HRS to assess, among other objectives:
- Ease of Surgical Implantation & Instrument Development
It was determined that the Reverse HRS surgical implantation could be performed using all standard surgical approaches. State-of-the-art instruments and practical tray layouts have been designed to further support ease of surgical implantation.
- Establish Biomechanical Equivalence
It was determined that the ratio of the abductor lever arm to gravitational lever arm was .51 to .53 and the force on the femur head was at 2.7 times body weight, all metrics were well within normal limits.
In addition to bench level testing, the Cadaver research results validated that, “The Reverse HRS has the same biomechanical characteristics as a normal hip and a well positioned THA”, Victor Frankel, M.D., PhD.
- Anatomical Range of Motion and Stability at normal and mal-positioned acetabular cup placement
In both normal and extreme mal-positioned acetabular cup positions, with various Flexion, Internal and External positions, the Reverse HRS was stable and did not impinge or dislocate. See chart below.
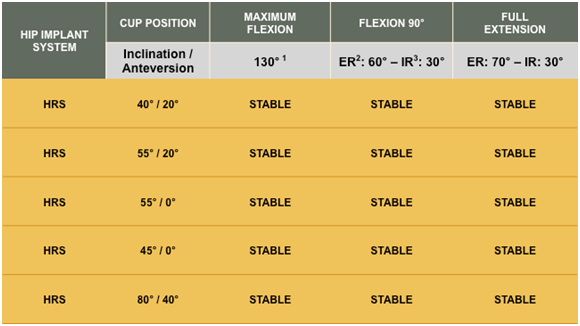
Surgeon Post Cadaver Study Performance Conclusions
All participating orthopaedic surgeons unanimously agreed with the following post study performance conclusions to be included in FDA Validation Feedback form:
- Variability in component positioning did not lead to instability, impingement or reduction of motion
- Validates forgiving design
- Instability did not occur even with gross acetabular component mal-position
- Component design allowed extreme ranges of motion
- Implantation can be performed with standard surgical approaches
Cadaver Research further validates the Reverse HRS forgiving design. The Reverse HRS maintained stability in acetabular cup malposition and at extended ranges of motion. The system did not impinge nor dislocate in standard or extreme component positions.

